



Under the FDA recognition, IAS has the authority to accredit third-party certification bodies, also known as third-party auditors. These certification bodies, once accredited, can conduct food safety audits and issue certifications of foreign food facilities (including farms) and the foods – both human and animal – that they produce. Those certifications can be used by importers to establish eligibility for participation in the Voluntary Qualified Importer Program (VQIP), and in certain circumstances FDA can require that imported products be certified before entering the United States.
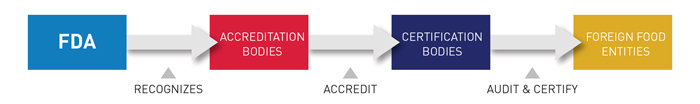
IAS is recognized by the U.S. FDA to accredit certification bodies with the ability to conduct food safety audits as they pertain to the following regulations:
Scope Expansion: Certification bodies that desire to expand their scope to include other areas may contact IAS.
Join the FDA Third-Party Certification Program List
Get the latest information about certification practices, standards, training courses and more, when you join the IAS list for FDA Third-Party Certification. To join, click here.
